Concept:
When water vapor in a frank is cooled and condensed into water drops, the pressure in the flask reduces greatly.Introduction:
When water vapor changes into water drops,its volume reduces to less than one thousandth.Using this principle, we can make a virtual vacuum and let a balloon be sucked into a flask.Materials:
- a flask
- a tripod
- a balloon
- a wire gauze
- a Bunsen/alcohol burner
- water
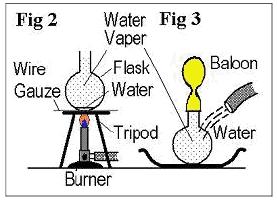
1. Put a little water in a flask. Place the flask on a wire gauze and heat it by Bunsen/alcohol burner
until water vapor begins to come out as shown in Fig.2.
2. When there is almost no water in the flask, stop heating and cover the mouth of the flask with the
mouth of balloon as shown in Fig.3.
3. Pour cold water around the bottom of the flask.
4. Observe what happens.
Science:
The volume of 1 mole water vapor at 100 ℃ is 30,600ml while that of 1
mole water at 100 ℃ is only 18ml. If we heat water until it comes to
boil, water vapor drives the air out of the flask. If we cool the flask
by pouring cold water around the flask after covering its mouth with a
balloon, water vapor in the flask is condensed into water drops and
there is little water vapor in the flask. So the pressure in the flask
also reduces greatly and the balloon is sucked into the flask.Things to do:
1. Find the way how to restore the balloon to its former state.2. Remove the shell of a boiled egg and prepare a bottle whose mouth is a little smaller than the egg.Observe what happens, when you put a burning match into the bottle and cover the mouth of the bottle with the naked egg. And find the reason.
source:Sucking a balloon into a flask





No comments:
Post a Comment